International Responses to Research on Valproate Risks
Resources produced by other countries on valproate. Regulation information is not relevant to Australia except for point of reference and comparison. Other information provides greater depth to the information available in Australia.
If links do not work please email brokenlink@valproate.info

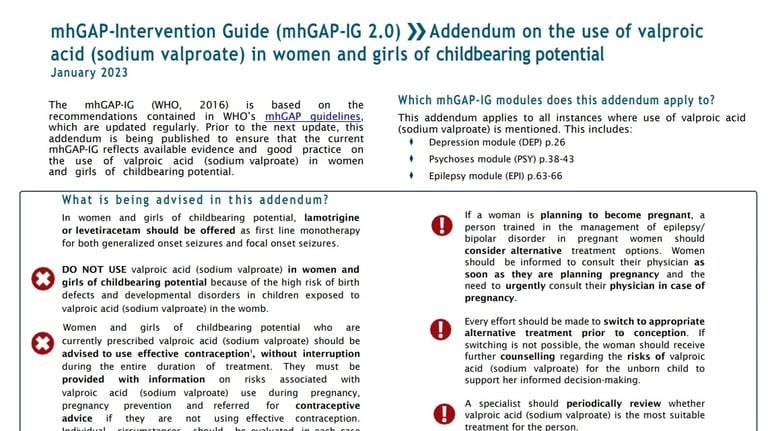
mhGAP INTERVENTION GUIDE
The EMA has undertaken considerable research and review of the impact and regulation of valproate. This link provides comprehensive information up to the 2018 restriction decisions that were then enforced in all EU countries.
Linked 2/12/2024
ADDENDUM TO mhGAP - VALPROATE SPECIFIC
Response to preliminary research that shows potential adverse outcomes for children conceived when father is taking valproate.
Linked 2/12/2024


WHO STATEMENT ON VALPROATE
The European Medicines Agency (EMA) raised the alarm internationally and drew attention to the need for strengthened warnings about valproate.
Linked 2/12/2024
World Health Organisation

EUROPEAN MEDICINES AGENCY 2018
The EMA has undertaken considerable research and review of the impact and regulation of valproate. This link provides comprehensive information up to the 2018 restriction decisions that were then enforced in all EU countries.
Linked 2/12/2024
EUROPEAN MEDICINES AGENCY 2024
Response to preliminary research that shows potential adverse outcomes for children conceived when father is taking valproate.
Linked 2/12/2024


EUROPEAN MEDICINES AGENCY 2014
The European Medicines Agency (EMA) raised the alarm internationally and drew attention to the need for strengthened warnings about valproate.
Linked 2/12/2024
European Union

MHRA (REGULATOR) GUIDANCE
The Medicines & Healthcare products Regulatory Agency guidance on valproate inclusive of historical versions. Also includes links to other materials and resources. Consumer-friendly website
Linked 4/12/2024
MHRA REVIEW OF EVIDENCE AND REGULATORY RESTRICTIONS 2023
The MHRA undertook a review of evidence of valproate harm and effectiveness of restrictions. Report includes a plain English summary.
Linked 4/12/2024

EPILEPSY SOCIETY UK INFORMATION ON VALPROATE
Information for people in the UK with epilepsy
Linked 9/12/2024
LONDON NHS VIDEO - VALPROATE & BIPOLAR DISORDER
NHS video: contains explanation on restrictions that are UK specific and important information on the risks of valproate for patients
Linked 9/12/2024
FIRST DO NO HARM - CUMBERLEGE REPORT
The report on the inquiry commissioned by the minister for health into the harms inflicted upon Britons by medical use of valproate, pelvic mesh & Primidos. The inquiry took 2 years and was led by Baronness Cumberlege who is a member of the House of Lords.
Linked 10/12/2024
HISTORICAL TIMELINE OF VALPROATE TO 2020
A useful detailed history of the regulation & use of valproate
Linked 10/12/2024
OPTIONS FOR REDRESS - THE HUGHES REPORT
The Office of the Patient Safety Commissioner was introduced in response to a recommendation arising from the First Do No Harm report. The Patient Safety Commissioner undertook a review to present options for redress for those harmed by valproate (also a recommendation from Baronness Cumberlege.)
Linked 10/12/2024
United Kingdom
LETTER SENT TO WOMEN & GIRLS PRESCRIBED VALPROATE 2021
To reduce the impact of occurrences of lack of information being provided to patients by doctors, the NHS contacted all patients and provided information directly.
Linked 10/12/2024

MEDSAFE ALERT - PRESCRIBING FOR WOMEN AND GIRLS
Indications & contraindications updated 2019. Advice for consumers, prescribers & pharmacists; useful links provided
Linked 9/12/2024
MEDSAFE ALERT FOR PEOPLE WHO CAN FATHER CHILDREN
Information for consumers and healthcare professionals with detail and links to additional information
Linked 9/12/2024
MEDSAFE ALERT COMMUNICATION - PREGNANCY
Trans-Tasman Early Warning System Alert (not published in Australia. Information for consumers and healthcare providers.
Linked 9/12/2024
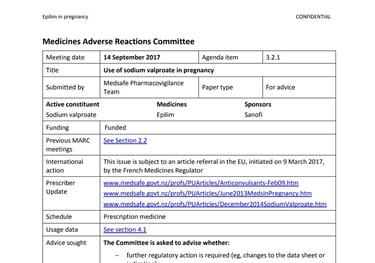
NZ MEDICATION ADVERSE REACTIONS COMMITTEE REVIEW REPORT 2017
Medsafe Pharmacovigilance Team referred valproate to the MARC in parallel action to the French referral to the EMA
Linked 9/12/2024
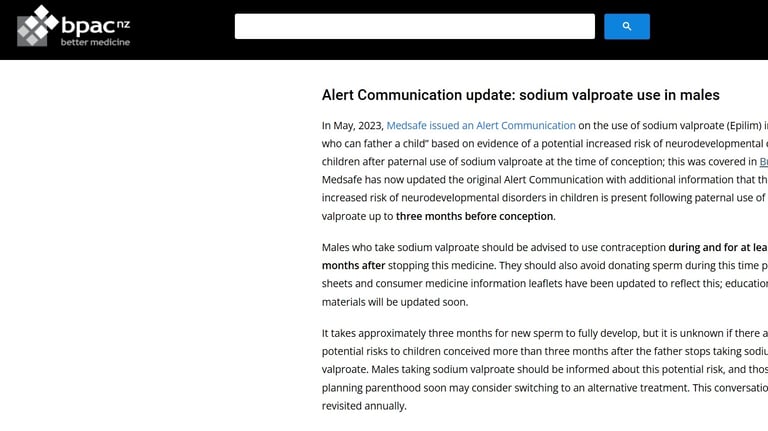
NZ BEST PRACTICE BULLETIN DEC 2023
Update on the risk associated with people who can father a child taking valproate
Linked 9/12/2024
New Zealand

SAUDI ARABIA GUIDELINE
Information for healthcare professionals, includes pregnancy prevention program
Linked 3/12/2024
SOUTH AFRICA ESSENTIAL MEDICINES LIST & TREATMENT GUIDELINES
Detailed treatment guidelines within the essential medicines list - addresses contraindications of valproate
Linked 4/12/2024
EGYPT - HEALTH CARE PROFESSIONALS INFORMATION
Egypt requires participation in a pregnancy prevention program inclusing risk acknowledgement forms. Information indicates that use for bipolar disorder is not recognised - the only indication int he information is epilepsy.
Linked 4/12/2024
Saudi Arabia, South Africa, Egypt

MALAYSIA PHARMACY BULLETIN 2019
Update for pharmacists on research and contraindication changes
Linked 2/12/2024
MALAYSIA REGULATORY UPDATE 2020
Ministry of Health National Pharmaceutical Regulatory Agency, includes pregnancy prevention program
Linked 2/12/2024
SINGAPORE ANNUAL REMINDER LETTER 2024
Annual letter to healthcare professionals to provide an update or reminder on risk minimisation measures.
Linked 4/12/2024
SINGAPORE REGULATORY UPDATE 2024
Singapore alert to updated regulatory information 2024
Linked 4/12/2024
SINGAPORE PATIENT GUIDE
The patient guide is in plain text and not readily available online without a specific search for the booklet
Linked 4/12/2024
Singapore:
Singapore has implemented active risk minimisation measures such as a pregnancy prevention program. However, very little information is available directly to the public with a strong emphasis on healthcare professional access only. Some information is online but unless a document is specifically known about to search, consumers will not see it.
Malaysia & Singapore

The USA and Japan have responded in a similar manner to Australia. Neither have placed any restrictions on prescribing.